Why it is challenging to achieve tight tolerances with semi-crystalline polymers such as POM, Nylon, PP, and PBT ?
Let us look into the difference between amorphous and crystalline structures and how does it affect the dimensional stability of a material when heated and cooled?
When heated, amorphous polymers retain their random molecular arrangement and do not undergo any significant change in their molecular structure. This makes it easier to achieve dimensional stability with amorphous polymers as their structure remains the same in both cool and heated conditions.
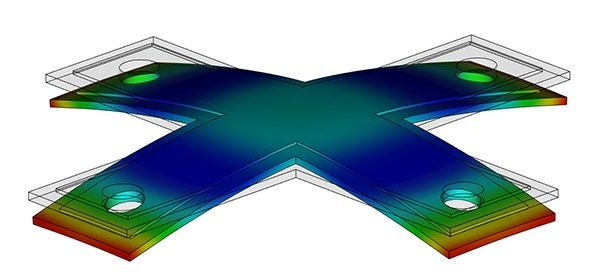
However, when semi-crystalline polymers are heated, their specific, bundle-shaped molecular structure starts to open up and become more randomly arranged like cotton. This phase-changing property makes it difficult to achieve dimensional stability with semi-crystalline polymers, as their molecular structure changes depending on whether they are in a cool or heated state. Additionally, when cooled, semi-crystalline polymers have to return to their original bundle-shaped molecular structure, which can result in significant shrinkage and warping.
Therefore, achieving tight tolerances with semi-crystalline polymers requires careful mold design and process control to account for their phase-changing behavior. This is particularly important for critical dimension parts, such as switch parts, where even small deviations in size can affect performance. Understanding the behavior of both amorphous and semi-crystalline polymers when heated is crucial for selecting the right polymer for a specific application and achieving optimal dimensional stability.